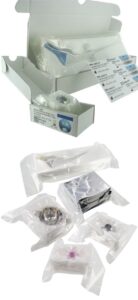
VacUpac is an Evolutis proprietary technology of vacuum packaging process for any sterile implant.
The development of VacUpac was adapted to the specific requirements of the orthopaedic industry, with major emphasis on the ease and the safety of use in a healthcare institution.
VacUpac enables:
- to protect implants during shipping and transport and when manipulated by the hospital staff
- to entirely reduce any micro-mobility of the implant in the packaging, reducing in proportion any abrasion of the implant and/or its coating with the blister packs / bags / foams
- to evidence and make visible the sterile state of the product as soon as the box is opened
- to facilitate the presentation and the grip of the product in the surgical theater
- to allow for a “no touch” handling of the implants
- to protect the implants from light and/or oxidation depending on their material
- to guarantee a constant integrity of the sterility throughout the duration of validity of the IMD
- to reduce the volume of total storage in the hospital and the volume of not recycled waste in an initiative of environmental preservation
VACUPAC AND PEXEL
The VacUpac technology is essential to the achievement and the preservation of the characteristics of the PEXEL polyethylene:
- The level of vacuum obtained when packaging under VacUpac being close to complete, during the crosslinking process of the PEXEL polyethylene(mini 40 mRad) the recombination of the free radicals with the oxygen atoms cannot be made. The free radicals finding no oxygen atom to combine with, will combine with another molecular chain of polyethylene and so contribute to the increase of the crosslinking and the final density of the material.
- The resistance of the multilayers pouches and of the seal over time guarantees the steadiness of the vacuum in the packaging during the entire life cycle of the IMD. The PEXEL polyethylene is not altered during its stocking before use, and its capacity to resist to wear and to oxidation is not conditioned by the length of time between the date of manufacture and the date of implantation.